AG Heber
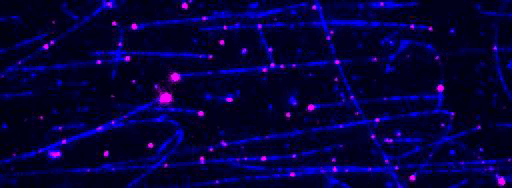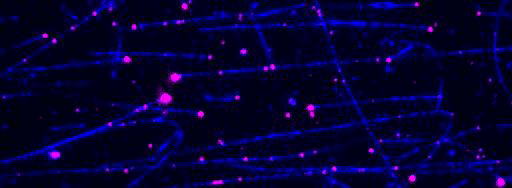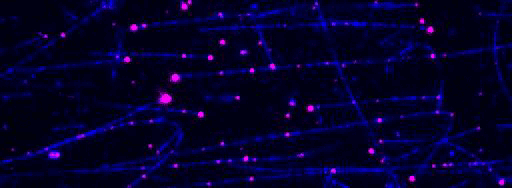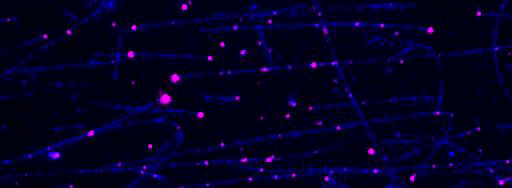
Kinesin-1 motor proteins moving on immobilized microtubules visualized by Total Internal Reflection Fluorescence (TIRF) microscopy.
Elucidating molecular motor-driven RNA localization through in vitro reconstitution
RNA localization followed by local translation is a wide-spread strategy to restrict gene expression to subcellular compartments, which is essential for many biological processes such as development, cell migration or nervous system function. Active transport of mRNA is carried out by members of three motor protein families: kinesins, dyneins and myosins. Kinesin and dynein provide long-range directional transport on the polarized microtubule (MT) network: kinesin typically translocates towards MT plus ends and dynein to MT minus ends. Class V myosins are responsible for short-range transport along randomly oriented actin filaments and have been implicated in anchoring of cargoes at their destination.
Many cargoes are transported bidirectionally along the microtubule cytoskeleton by dyneins and kinesins. Often, both motors are constantly bound to the cargo, facilitating fast switches of directionality or linking of subsequent transport steps. How motor activity is regulated in such complexes to prevent a futile tug of war remains puzzling. Even less is known about the interactions between the microtubule motors and the actin motors, myosins.
oskar RNA localization: A paradigm to study RNA transport
oskar mRNA localization in the Drosophila egg chamber is a paradigm for the study of RNA transport and motor regulation. For its correct localization, oskar requires the concerted actions of dynein, kinesin and MyosinV, making it an ideal system for studies of the interaction between MT and actin motors.
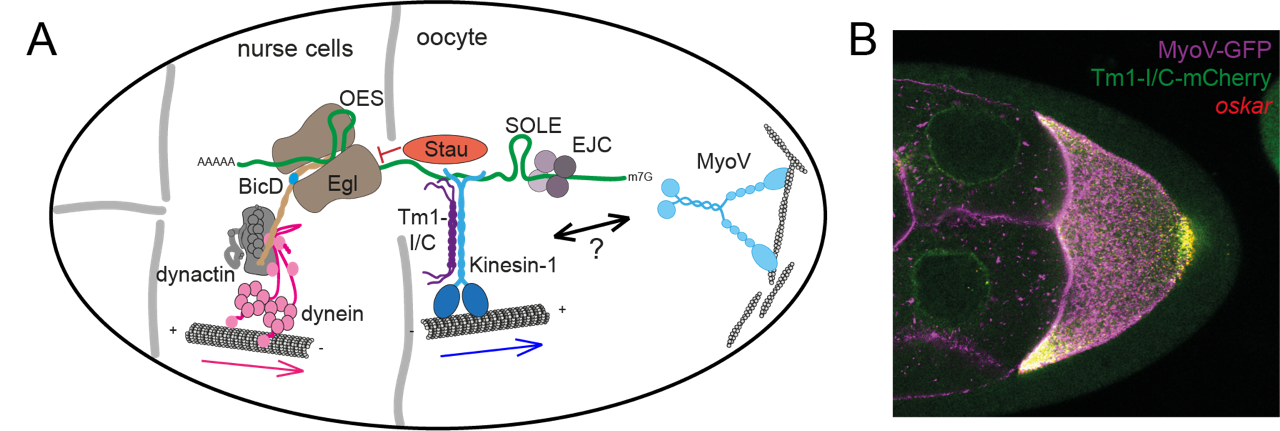
Figure 1: The localization of oskar mRNA in the Drosophila germline syncytium from the nurse cells to the oocyte posterior requires the consecutive action of dynein, kinesin-1 and MyosinV. A: Overview of the know components of the oskar transport machinery. In early stages of oogenesis, oskar mRNA is transported into the oocyte by dynein in complex with dynactin and the adaptor proteins BicD and Egl, which recognize the oocyte entry signal (OES) in the oskar 3’UTR. During mid-oogenesis, kinesin-1, together with the regulatory protein Tm1-I/C, translocates oskar towards MT plus ends at the posterior pole of the oocyte. This kinesin-mediated transport relies on the cis-acting spliced oskar localization element (SOLE), and deposition of the exon junction complex, as well as the dsRNA-binding protein Stau, which inhibits dynein by outcompeting Egl from the complex. B: Confocal fluorescence microscopy shows oskar mRNA, the kinesin-adaptor Tm1-I/C and the actin motor MyosinV in a stage 8/9 Drosophila oocyte.
The Drosophila egg chamber contains a germline syncytium, in which the oocyte is transcriptionally quiescent and relies on mRNAs provided by the 15 interconnected nurse cells for its development. Localization of oskar to the posterior pole of the developing oocyte is essential for abdominal patterning and germline formation in the embryo. Transport of oskar mRNA to this site occurs in two transport steps which require the successive activities of dynein and kinesin-1, with a timed motor switching event. In addition, oskar localization depends on actin-based MyosinV, which counterbalances kinesin-1 at the posterior cortex to anchor oskar at its destination.
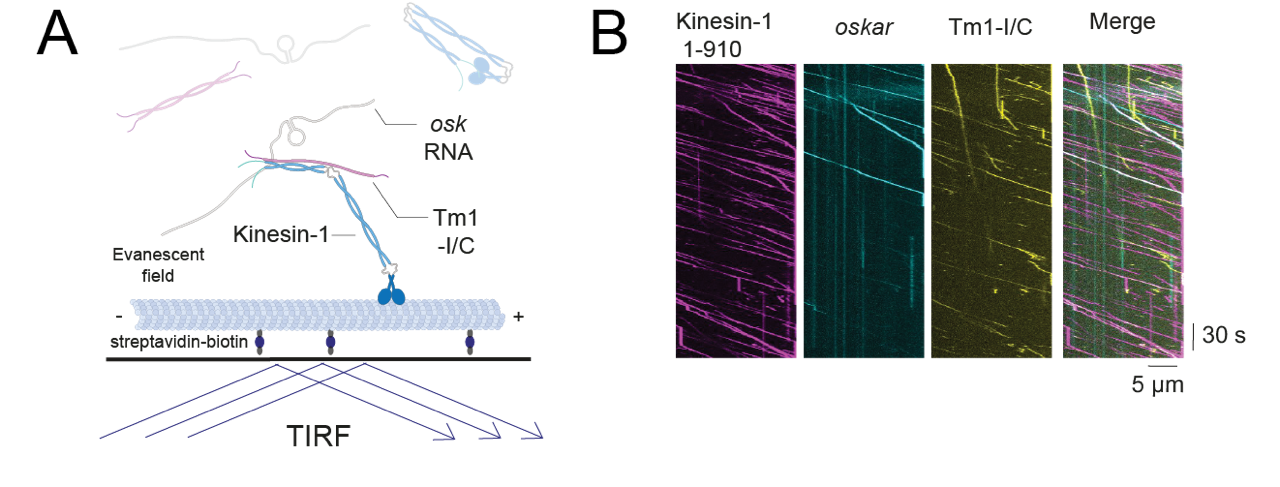
Figure 2: TIRF microscopy-based tracking of single transport particles. A: Assay scheme. B: Kymographs showing all movements of components of the transport particle on a microtubule.
We study oskar RNP transport structurally and functionally by in vitro reconstitution, using TIRF microscopy-based single transport particle tracking on immobilized MTs, and biophysical and structural characterization (NMR, SAXS, cryoEM) of the protein complexes involved. The Drosophila egg chamber is a genetically tractable system, allowing us to validate our findings in vivo in transgenic fruit flies to complement our in vitro studies. With this approach, we have uncovered details of the regulatory mechanisms underlying the motor switch from dynein to kinesin-1 (Heber et al. 2024, Gaspar et al., 2023, Dimitrova-Paternoga et al., 2021). We are now tackling the interplay between the microtubule motors and MyosinV.
Why is this important for humanity?
RNA localization occurs in virtually all cell types and domains of life. It is especially important in big and asymmetric cell types, such as neurons. The human nervous system relies on mRNA transport for its development but also for its adult function, for example to fine-tune synapses. This is of special interest for understanding neurological disorders. Neuronal mRNA transport granules depend on microtubule and actin-based motors, such as oskar, but contain even more components and their composition is much less understood. Looking at oskar, we can unravel general molecular mechanisms underlying transport by multiple motors.
